US FDA Confirms Toxic Cough Syrups Linked to Child Deaths in India Not Imported to United States
- Date & Time:
- |
- Views: 17
- |
- From: India News Bull
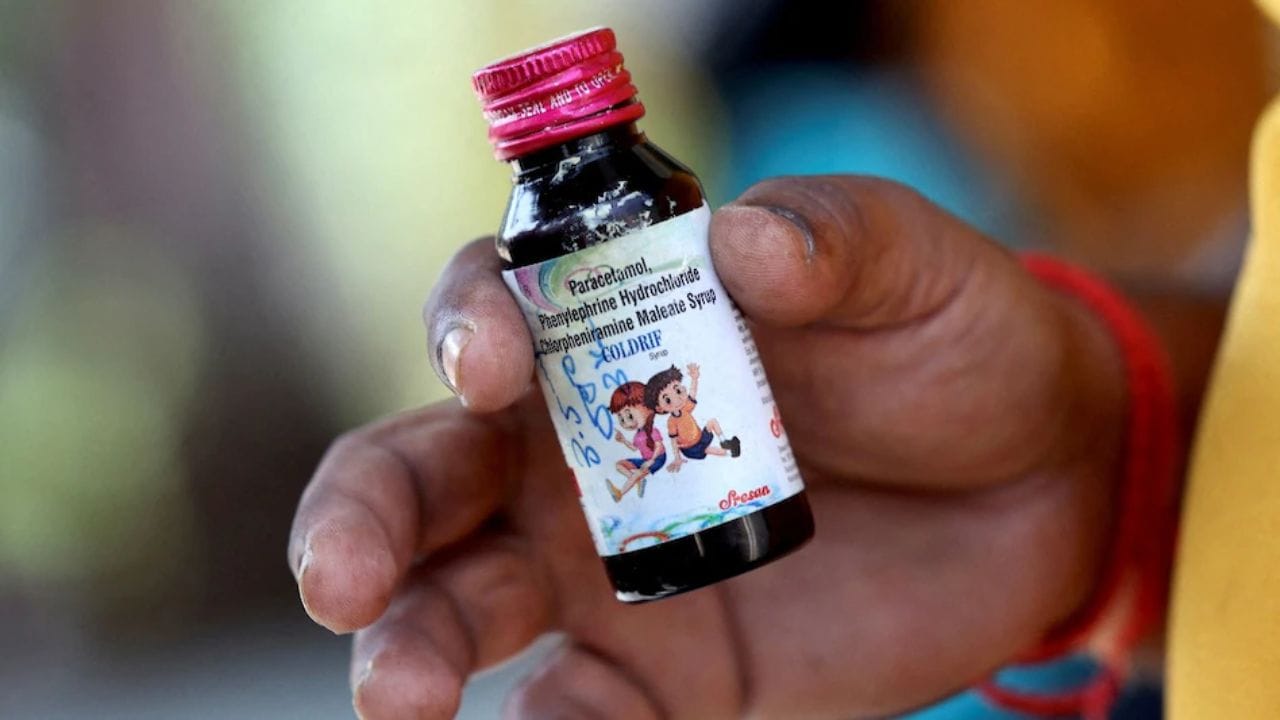
Numerous children in India have died within the past month following alleged consumption of contaminated cough syrup.
The US Food and Drug Administration issued a confirmation on Friday that the toxic cough syrups connected to children's fatalities in India had not been exported to the United States.
According to the World Health Organization, India suffers from a "regulatory gap" in the screening process for locally marketed syrup medications.
The US FDA stated they are cognizant of news reports regarding the devastating and ongoing contamination of children's cough and cold medicines in India with diethylene glycol and ethylene glycol.
Indian authorities issued public warnings against two additional cough syrup brands on Wednesday following the deaths of 17 children under five years of age, which were linked to toxic ingredients.
The children died in India during the past month after consuming cough medication containing toxic diethylene glycol in amounts nearly 500 times above the permissible limit, according to officials. All deaths were connected to the Coldrif medicine brand, which authorities banned after testing confirmed the presence of the chemical on October 2.
India's health regulatory body, the Central Drugs Standard Control Organization, informed the US regulator that these contaminated products were not exported from India to any other country, according to the FDA.
The FDA further emphasized its continued vigilance in preventing contaminated medications from entering the United States and urged manufacturers to ensure that drugs marketed in the US maintain the highest quality and safety standards.
Source: https://www.ndtv.com/world-news/cough-syrup-coldrif-linked-to-child-deaths-in-india-not-shipped-to-us-drug-regulator-9435149