Investigation Reveals Safety Lapses and Regulatory Failures in Fatal Cough Syrup Contamination Case
- Date & Time:
- |
- Views: 13
- |
- From: India News Bull
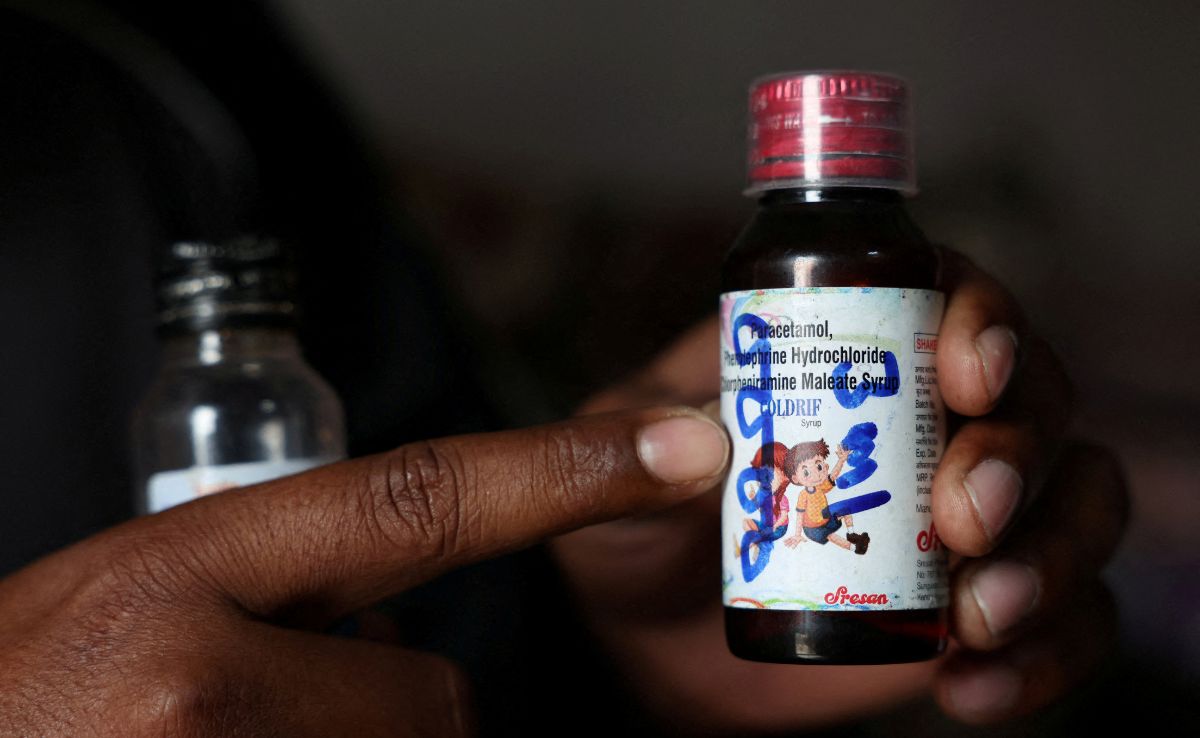
Officials are investigating potential safety lapses in pharmaceutical ingredient supply that may have led to contaminated cough syrup responsible for at least 24 children's deaths in recent months, according to three individuals familiar with the situation.
Three health and drug safety officials from Tamil Nadu informed Reuters they believe the solvent used in producing a batch of Coldrif cough syrup may have been contaminated with toxic chemicals during its supply to the manufacturer, Sresan Pharmaceutical Manufacturer.
Sresan acquired 50 kg of propylene glycol (PG) solvent from local chemicals distributor Sunrise Biotech on March 25. Sunrise had purchased it that same day from Jinkushal Aroma, a small company that produces fragrance blends for liquid detergents and other chemicals, according to supplier interviews and an October 3 investigation report by Tamil Nadu's pharmaceutical regulator, exclusively reviewed by Reuters.
The Tamil Nadu Drugs Control Department did not respond to multiple requests for comment regarding their investigation.
Authorities have reported that Coldrif syrup was heavily contaminated with diethylene glycol (DEG), a known industrial toxin. They are investigating how this chemical was introduced into the solvent, which serves as a base for dissolving active ingredients in cough syrup.
The fatalities, which began in September, have renewed concerns about safety standards in India's $50 billion pharmaceutical sector, previously tarnished by over 140 children's deaths in Africa and Central Asia during 2022 and 2023 from Indian-made cough syrups containing contaminated solvents.
Following those earlier deaths, New Delhi had committed to enhancing quality controls.
Health officials indicate that DEG is sometimes fraudulently or inadvertently used in medicines as a substitute for the more expensive PG. High-level consumption of DEG has been linked to acute kidney damage and death in children.
Reuters is revealing for the first time details about the investigation's focus and breaches in global pharmaceutical safety practices in the chemical delivery process to Sresan.
Sresan's manufacturing license has been revoked and its founder G. Ranganathan is in custody. Attempts to contact representatives at Sresan's corporate office and Ranganathan's residence were unsuccessful. Reuters could not identify a legal representative for Ranganathan.
The Central Drugs Standard Control Organisation, which oversees pharmaceuticals at the federal level, directed inquiries to the health ministry, which referred Reuters to a government statement indicating increased inspections of drug facilities and review of pediatric cough syrup usage.
Chemical manufacturers typically deliver PG solvents in sealed containers to prevent contamination, but Sunrise confirmed to Reuters that they had repackaged the solvent without proper sealing before delivery.
The Drugs and Cosmetics Act prohibits the sale and handling of pharmaceutical-grade ingredients like medicinal PG by entities without proper drug licenses.
Neither Jinkushal nor Sunrise possess licenses for handling pharmaceutical-grade ingredients, as confirmed by both wholesale distributors to Reuters. Their owners stated they were unaware the PG they sold would be used in medication production.
Both Sunrise operator Vipul Jain and Jinkushal owner Jitender Vishwakarma denied handling highly toxic DEG and claimed no knowledge of how the solvent might have become contaminated.
Reuters could not establish the contamination's origin or responsible parties.
Inspections conducted by state regulators following the deaths identified hundreds of "critical" and "major" violations at Sresan's factory outside Chennai, including product storage in "unhygienic conditions" and "falsification of data," according to the October 3 report. However, the regulator did not directly link these breaches to the deaths.
The PG that Sresan reported using was produced by South Korean manufacturer SK picglobal, according to a certificate of analysis shared with investigators by Jinkushal and Sunrise, which Reuters reviewed.
The certificate detailed the PG's manufacturing date and chemical composition. While Reuters couldn't independently verify the content accuracy, an SK picglobal spokesperson indicated that the copy reviewed appeared authentic.
SK picglobal exported the solvent in a sealed 215 kg barrel to an Indian distributor, who subsequently sold it to Jinkushal.
Vishwakarma of Jinkushal stated that he broke the seals and repackaged the solvent in his store before selling a portion to chemicals distributor Sunrise.
Jain of Sunrise confirmed transporting the chemical to Sresan in two containers without tamper-proof seals, as the drugmaker had not requested the entire original 215 kg supply.
The SK picglobal spokesperson emphasized that the company strictly prohibits repacking or redistribution of its products.
When asked if such restrictions were included in sales contracts, the company mentioned informing customers during meetings that it doesn't "guarantee the quality of products that have been re-packed or arbitrarily subdivided for sale," without providing additional details.
Sresan had previously faced penalties from authorities, with its founder imprisoned for one day in both 2020 and 2022 due to product concerns, before receiving fines, according to four officials familiar with the matter, though they provided no documentary evidence for these penalties.
Tamil Nadu's health minister informed lawmakers last month that Sresan had been penalized in 2021 and 2023 for "minor violations," without specifying further.
Despite Sresan's record and regulations requiring annual inspections, the drugmaker's factory had not been inspected since 2023, according to two state health officials.
Reuters could not independently confirm when Sresan had last been inspected prior to the deaths.
Source: https://www.ndtv.com/india-news/safety-lapses-weak-oversight-being-probed-into-cough-syrup-deaths-report-9673850