toxic cough syrup
WHO Warns India Must Strengthen Regulations to Prevent Toxic Cough Syrup Deaths
Oct 21, 2025 08:21 pm CST
Despite some regulatory improvements, India still faces significant challenges in preventing toxic cough syrup sales, according to the WHO. Following the deaths of 24 children linked to contaminated medicine containing dangerous levels of diethylene glycol, officials highlight the need for comprehensive testing requirements for both exported and domestically sold pharmaceutical products.
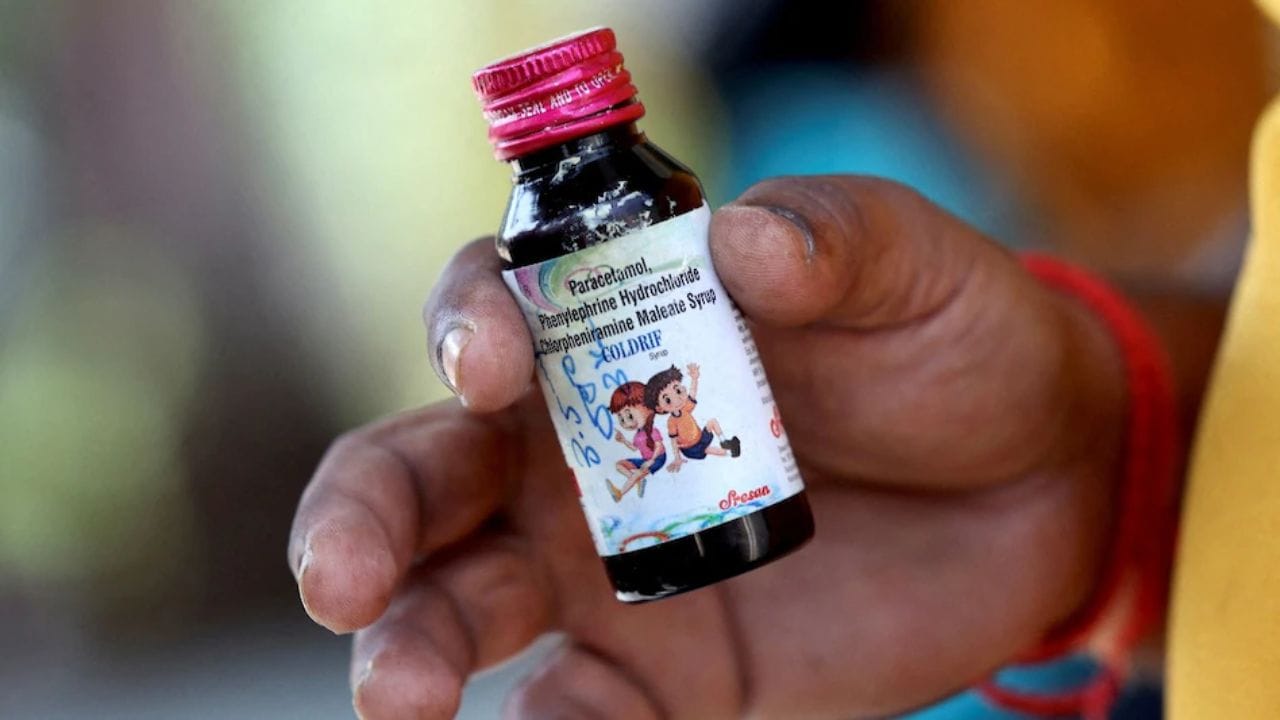
ED Raids Premises of Pharmaceutical Firm Linked to Fatal Cough Syrup: 20 Children Dead from Contaminated Medication
Oct 13, 2025 12:14 pm CST
The Enforcement Directorate has raided multiple premises of Sresan Pharmaceuticals, manufacturer of 'Coldrif' cough syrup that contained toxic diethylene glycol and caused at least 20 children's deaths. The investigation revealed over 350 violations at the company's facility, prompting state bans and WHO concerns about India's regulatory gaps in drug safety screening.
US FDA Confirms Toxic Cough Syrups Linked to Child Deaths in India Not Imported to United States
Oct 11, 2025 09:44 am CST
The US Food and Drug Administration has confirmed that toxic cough syrups responsible for 17 children's deaths in India were not exported to the United States. Indian authorities banned the Coldrif brand after tests revealed diethylene glycol levels 500 times above the permissible limit, while the WHO highlighted India's "regulatory gap" in screening medications sold locally.
Deadly Cough Syrup Scandal: 23 Children Die as CAG Report Reveals Systemic Failures in Drug Testing
Oct 10, 2025 04:06 pm CST
A CAG audit has revealed critical failures in drug testing procedures following the deaths of 23 children in Madhya Pradesh from toxic cough syrup. The report highlights significant shortfalls in Tamil Nadu's pharmaceutical inspection system, where manufacturer Sresan Pharmaceuticals was found adding dangerous levels of diethylene glycol to their Coldrif syrup, far exceeding safety limits. This regulatory negligence has sparked a heated blame exchange between state governments.
WHO Warns of Regulatory Gaps After Fatal Cough Syrup Contamination in India
Oct 09, 2025 01:10 pm CST
The World Health Organization has raised concerns about India's regulatory gaps in screening for toxic chemicals in medicines after contaminated cough syrups caused the deaths of 20 children in Madhya Pradesh and Rajasthan. WHO highlighted the potential international risk of such products being exported through unregulated channels, following the discovery of high levels of diethylene glycol in several cough syrup brands.
Deadly Negligence: How 20 Children Died from Toxic Cough Syrup While Madhya Pradesh's Drug Testing System Failed
Oct 08, 2025 11:51 pm CST
In Madhya Pradesh, India, a catastrophic breakdown in the drug regulatory system resulted in 20 children's deaths from toxic cough syrup Coldrif. This tragedy exposes critical failures including drug samples sent by ordinary post, delayed testing, premature safety declarations, and an overwhelmed infrastructure with unused mobile testing units. While Tamil Nadu acted swiftly to ban the dangerous medication, Madhya Pradesh's bureaucratic delays allowed the toxic syrup to claim more lives, revealing systemic issues that remain unresolved even as seven children continue fighting for survival.
Deadly Contamination: Madhya Pradesh Bans Coldrif Cough Syrup After 10 Deaths and Confirmed Diethylene Glycol Presence
Oct 04, 2025 05:16 pm CST
The Madhya Pradesh government has banned 'Coldrif' cough syrup after an NDTV report revealed it contained 48.6% Diethylene Glycol, a toxic industrial solvent. Ten deaths have been linked to the medication, with affected children showing symptoms consistent with DEG poisoning. All products from manufacturer Sresan Pharmaceuticals have been suspended pending investigation.