ED Raids Premises of Pharmaceutical Firm Linked to Fatal Cough Syrup: 20 Children Dead from Contaminated Medication
- Date & Time:
- |
- Views: 11
- |
- From: India News Bull
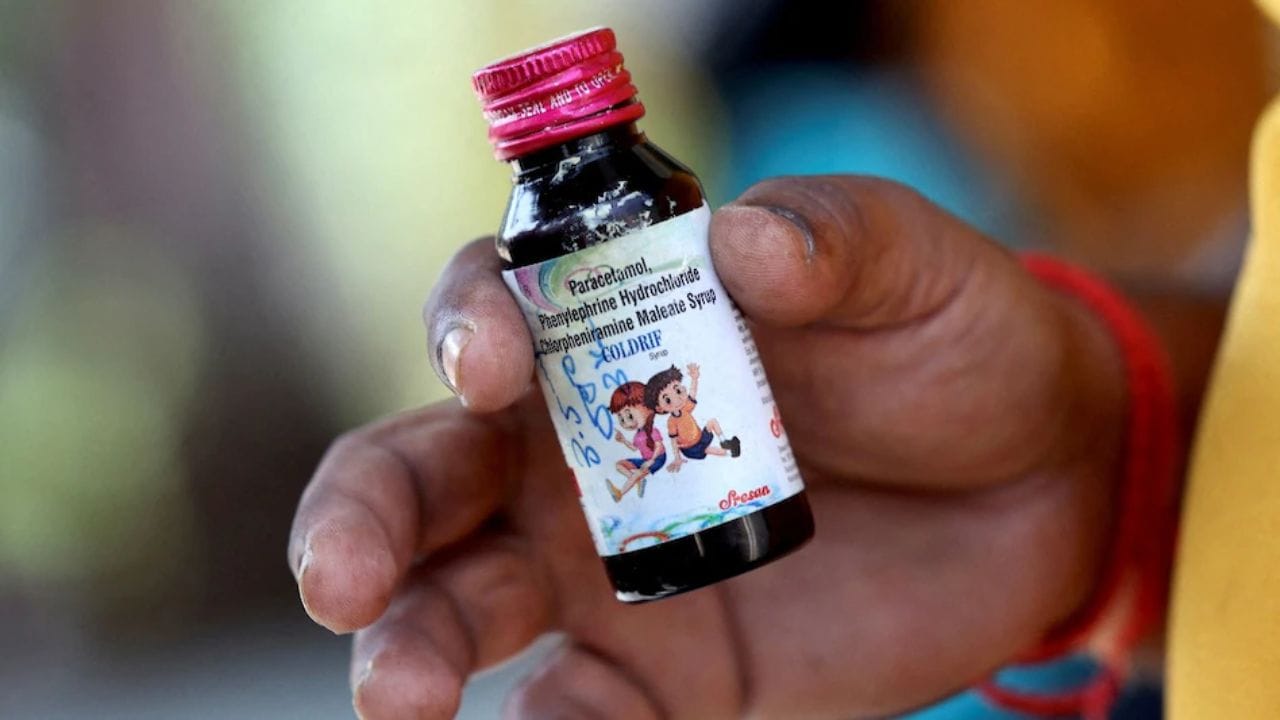
The Enforcement Directorate is currently conducting searches across seven locations in Chennai connected to Sresan Pharmaceuticals, the manufacturer of the toxic 'Coldrif' cough syrup that has reportedly caused at least 20 children's deaths in Madhya Pradesh and Rajasthan.
Officials confirm that the probe agency has filed a money laundering case against the pharmaceutical company and is also searching the homes of senior officials from Tamil Nadu's drug control office.
Ranganathan Govindan, the owner of Sresan Pharmaceuticals, was apprehended last week and placed in police custody for 10 days in relation to this case.
Investigations revealed that the 'Coldrif' syrup contained dangerous levels of diethylene glycol (DEG), a highly toxic substance. Reports indicate that over 350 violations were discovered at the company's manufacturing facility in Tamil Nadu, with 38 of these being classified as serious breaches.
Despite its inadequate infrastructure and numerous violations of national drug safety regulations, the Kanchipuram-based pharmaceutical company continued operations for more than a decade after receiving its license from the Tamil Nadu Food and Drug Administration (TNFDA) in 2011. Officials clarified that while the license was renewed in 2016 by the Tamil Nadu FDA, the Central Drugs Standard Control Organisation (CDSCO) was not involved in this process.
Multiple states including Madhya Pradesh, Rajasthan, Tamil Nadu, and Delhi have now prohibited the distribution of the cough syrup. The Tamil Nadu government has ordered the company to cease operations and is considering permanent revocation of its manufacturing license.
In response to the nationwide outrage over these child fatalities, the World Health Organisation (WHO) has expressed serious concerns regarding the "regulatory gap" in testing for diethylene glycol and ethylene glycol in domestically marketed medicines in India. The organization also highlighted the potential risk of contaminated products being exported to other countries.
The WHO statement emphasized concerns about "the potential risk of contaminated products being exported to other countries, particularly via unregulated channels," as well as "the regulatory gap in DEG/EG screening for domestically marketed medicines in India" and the importance of "identifying the source of the contamination and identifying and removing any contaminated pharmaceutical material which may be in circulation."
The organization has reportedly contacted the CDSCO seeking clarification about the contaminated medicines.
Source: https://www.ndtv.com/india-news/sresan-pharma-probe-agency-ed-raids-premises-linked-to-pharma-firm-behind-killer-cough-syrup-coldrif-9443845