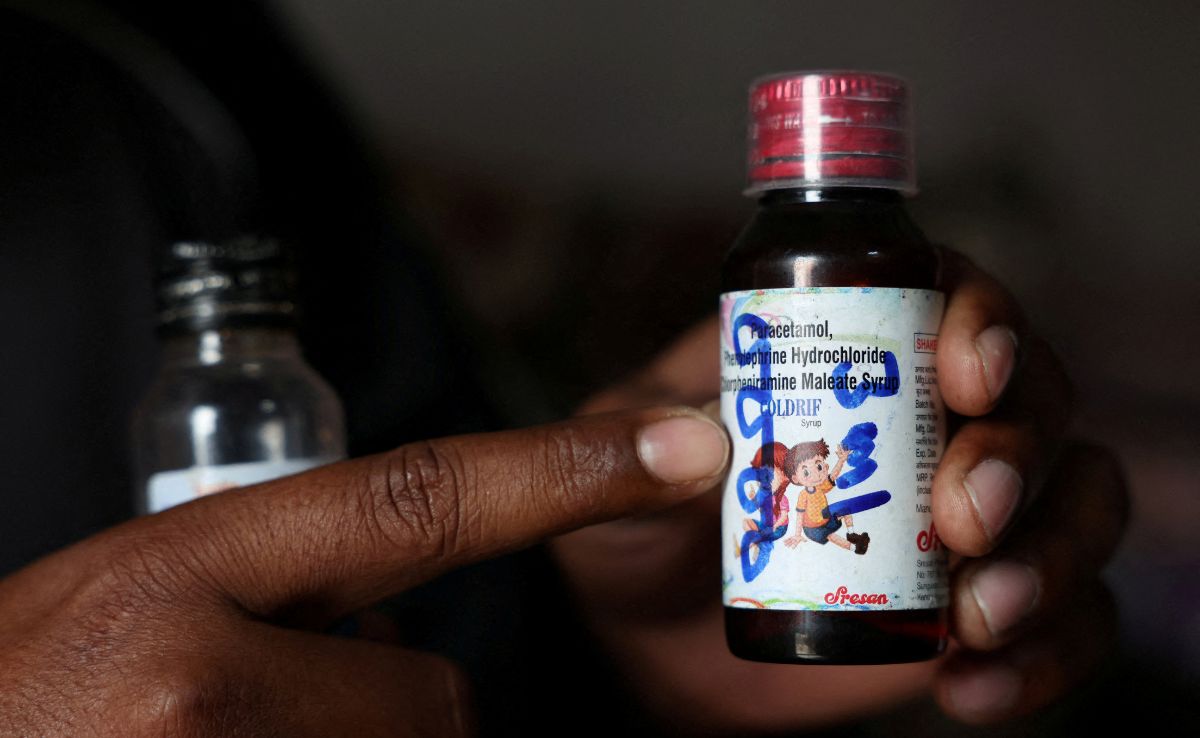
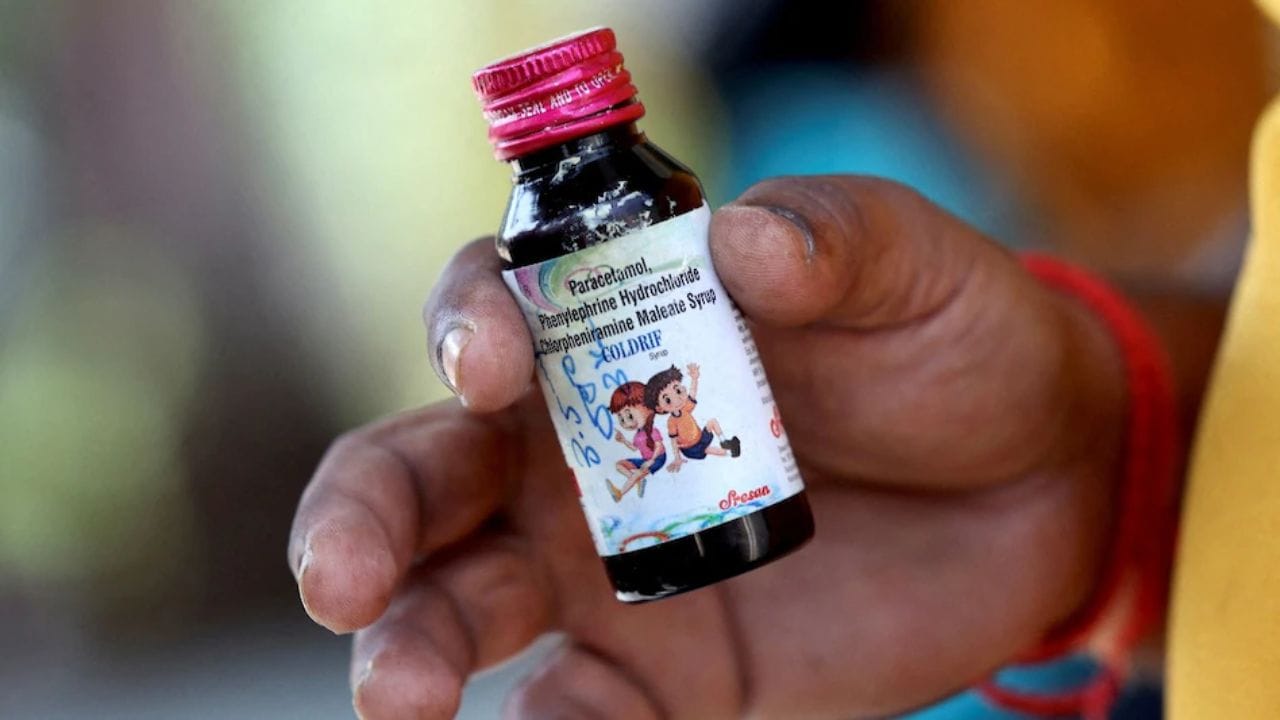
Coldrif syrup
Investigation Reveals Safety Lapses and Regulatory Failures in Fatal Cough Syrup Contamination Case
Nov 21, 2025 04:14 pm CST
An exclusive investigation into the deaths of 24 children from contaminated cough syrup has uncovered serious safety breaches in India's pharmaceutical supply chain. The report details how improper handling of propylene glycol solvent, unauthorized repackaging by unlicensed distributors, and inadequate regulatory oversight may have allowed toxic diethylene glycol to contaminate Coldrif cough syrup, raising new concerns about quality control in India's $50 billion pharmaceutical industry.
Contaminated Well Water with Dead Pigeons Sickens 60 in Madhya Pradesh: Health Crisis Update
Oct 16, 2025 05:32 pm CST
In Madhya Pradesh's Rajola village, 60 people have fallen ill after consuming water from a well contaminated by dead pigeons. Officials have closed the well and established medical camps for the affected residents. This incident occurs amid broader health concerns in the region, including recent child deaths linked to cough syrup that have prompted strengthened drug monitoring regulations.
ED Raids Premises of Pharmaceutical Firm Linked to Fatal Cough Syrup: 20 Children Dead from Contaminated Medication
Oct 13, 2025 12:14 pm CST
The Enforcement Directorate has raided multiple premises of Sresan Pharmaceuticals, manufacturer of 'Coldrif' cough syrup that contained toxic diethylene glycol and caused at least 20 children's deaths. The investigation revealed over 350 violations at the company's facility, prompting state bans and WHO concerns about India's regulatory gaps in drug safety screening.
CAG Report Reveals Madhya Pradesh's Procurement of Banned Drugs Amid Fatal Cough Syrup Incident
Oct 10, 2025 11:21 pm CST
A recent CAG audit has revealed that Madhya Pradesh's public health corporation procured banned medications worth Rs 1.8 crore between 2017-2022, despite central prohibitions. This regulatory failure has gained heightened scrutiny following the deaths of 23 children from toxic cough syrup in Chhindwara, raising serious concerns about the state's pharmaceutical safety protocols and oversight mechanisms.
364 Violations Discovered at Sresan Pharma Following Deadly Cough Syrup Incident: DEG Contamination Claims 20 Children's Lives
Oct 09, 2025 09:04 pm CST
An investigation into Sresan Pharma revealed 364 violations at their Tamil Nadu facility following a tragic incident where contaminated cough syrup containing excessive diethylene glycol resulted in at least 20 child fatalities in Madhya Pradesh. The CDSCO is now launching nationwide inspections of cough syrup manufacturers while several states implement stricter regulations for pediatric cough medication dispensing.
Deadly Cough Syrup Scandal: Pharmaceutical Owner Arrested in Madhya Pradesh Child Deaths Case
Oct 09, 2025 04:24 pm CST
The owner of Sresan Pharma, Ranganathan Govindan, has been arrested in Chennai following the deaths of 20 children in Chhindwara, Madhya Pradesh, who consumed toxic Coldrif cough syrup. Police conducted a meticulously planned operation to apprehend the suspect, who had been evading authorities since the tragedy came to light. Investigations reveal the company was operating despite being struck off official registers, raising serious questions about regulatory oversight in India's pharmaceutical industry.
India's Drug Regulator Acknowledges Pharmaceutical Manufacturing Lapses Following Fatal Cough Syrup Incidents
Oct 09, 2025 12:51 am CST
India's top drug regulator has admitted serious deficiencies in pharmaceutical manufacturing practices after children died from suspected contaminated cough syrups. CDSCO investigations revealed manufacturers failing to test raw materials and finished products as required by law, leading to stricter enforcement measures nationwide. The scandal has damaged India's reputation as a global pharmaceutical supplier, with multiple states implementing emergency restrictions on cough medication distribution.
Deadly Contamination: How Substandard Manufacturing Led to Fatal Cough Syrup Poisoning of 16 Children
Oct 06, 2025 10:50 pm CST
An investigation into the fatal Coldrif cough syrup reveals shocking manufacturing conditions at Sresan Pharmaceuticals, where industrial chemicals replaced pharmaceutical-grade ingredients, leading to 16 children's deaths in Madhya Pradesh. Inspectors found 39 critical and 325 major violations, including untested raw materials, untrained staff, and toxic levels of diethylene glycol—nearly 500 times above permissible limits.
Deadly Cough Syrup Tragedy: Families Lose Children and Livelihoods in Madhya Pradesh
Oct 06, 2025 02:28 am CST
Families in Madhya Pradesh are devastated after 11 children died from contaminated Coldrif Cough Syrup, causing kidney failure. Parents sold homes, vehicles, and mortgaged land to save their children, only to face heartbreak and financial ruin. Despite government compensation of Rs 4 lakh per victim, bereaved families demand justice over financial aid as they struggle with profound loss and emptied savings.
India's Health Ministry Responds to Child Deaths from Contaminated Cough Syrup: Urgent Regulatory Actions and Safety Measures
Oct 05, 2025 11:15 pm CST
Following the deaths of 14 children in Madhya Pradesh and Rajasthan linked to contaminated cough syrup, India's Health Ministry has implemented emergency measures to enhance pharmaceutical oversight. The tainted Coldrif cough syrup contained diethylene glycol, prompting stricter enforcement of manufacturing standards, rational prescription practices for pediatric medications, and improved surveillance systems. Health authorities are warning of criminal negligence in pharmaceutical production while emphasizing that most childhood coughs don't require medication.