pharmaceutical regulations
Tamil Nadu Authorities Revoke License of Pharmaceutical Firm Linked to Fatal Cough Syrup Cases
Oct 14, 2025 12:31 am CST
The Tamil Nadu Drug Control department has revoked Sresan Pharmaceuticals' manufacturing license following the discovery of over 350 serious violations at their facility. Their 'Coldrif' cough syrup, contaminated with diethylene glycol, caused multiple child deaths in Madhya Pradesh and Rajasthan. The Enforcement Directorate has launched raids at seven Chennai locations as investigations expand into potential financial irregularities related to the distribution of the toxic medication.
Deadly Cough Syrup Tragedy: How Tamil Nadu Regulatory Failures Led to 23 Children's Deaths
Oct 11, 2025 02:32 pm CST
An investigation by India's Central Drugs Standard Control Organisation reveals critical regulatory failures by Tamil Nadu authorities that allowed a pharmaceutical company to produce toxic cough syrup containing 48% diethylene glycol, resulting in the deaths of 23 children in Madhya Pradesh. The manufacturer operated for decades without proper certification, audits, or registration on mandatory regulatory portals.
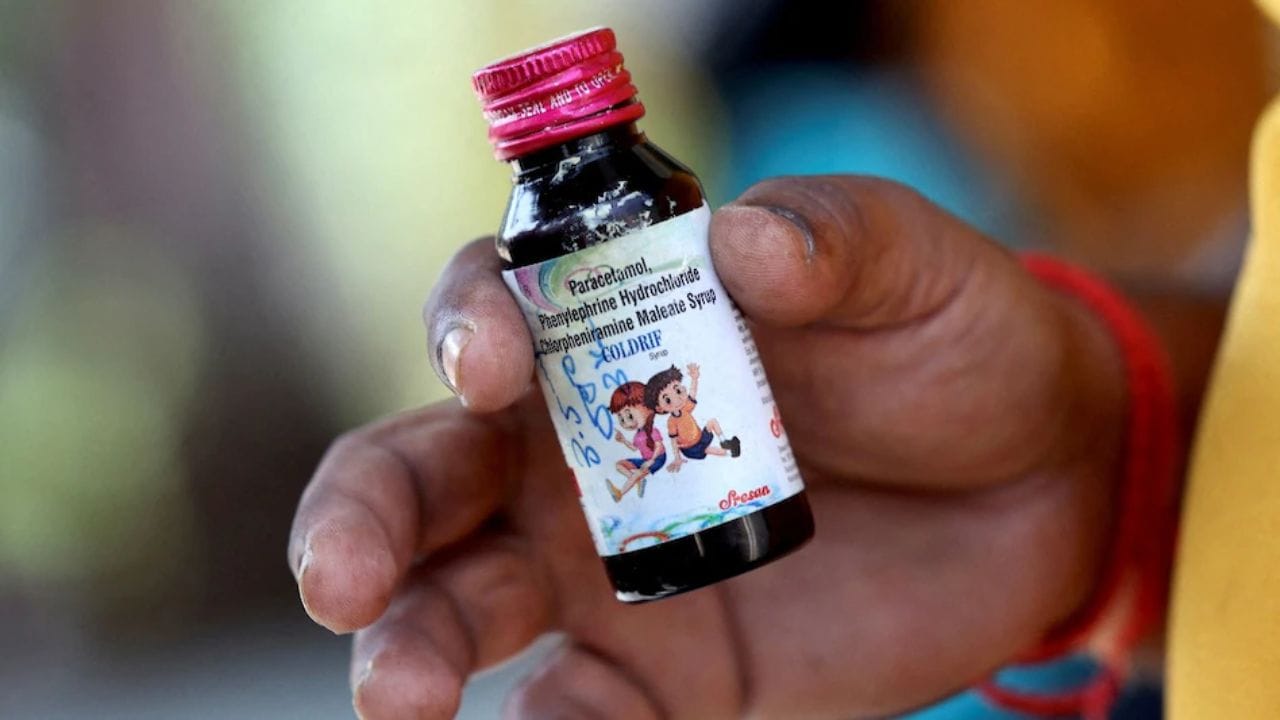
Coldrif Cough Syrup Manufacturer Arrested: Owner Charged in Fatal Adulteration Case Linked to Children's Deaths
Oct 09, 2025 01:36 pm CST
The owner of Sresan Pharmaceuticals, Ranganathan Govindan, has been arrested for manufacturing adulterated Coldrif cough syrup linked to at least 20 children's deaths across multiple Indian states. Investigations revealed the syrup contained dangerous levels of diethylene glycol (DEG), a toxic substance used in printing ink and glue manufacturing, at 46-48% against the permitted 0.1%. Nine states have banned the product as authorities expand their investigation into the deadly pharmaceutical scandal.
India's Drug Regulator Issues Critical Advisory Following Fatal Cough Syrup Contamination Cases
Oct 09, 2025 12:51 am CST
India's drug regulator has issued an urgent advisory highlighting serious lapses in pharmaceutical manufacturing practices after multiple child deaths linked to contaminated cough syrups. Inspections revealed manufacturers failing to test raw materials and finished products as required by law, prompting increased regulatory scrutiny across states and renewed concerns about India's pharmaceutical quality control systems.
Kerala Implements Strict Prescription Requirement for All Children's Medicines Following Cough Syrup Concerns
Oct 06, 2025 08:47 pm CST
The Kerala Health Department has mandated that all medicines for children under 12 require a doctor's prescription, responding to nationwide concerns over toxic cough syrups. An expert panel will study pediatric cough medicine safety while officials confirm no related incidents have occurred within Kerala. The state has suspended specific problematic batches and launched awareness campaigns to protect children's health through proper medication protocols.
India's Health Ministry Responds to Child Deaths from Contaminated Cough Syrup: Urgent Regulatory Actions and Safety Measures
Oct 05, 2025 11:15 pm CST
Following the deaths of 14 children in Madhya Pradesh and Rajasthan linked to contaminated cough syrup, India's Health Ministry has implemented emergency measures to enhance pharmaceutical oversight. The tainted Coldrif cough syrup contained diethylene glycol, prompting stricter enforcement of manufacturing standards, rational prescription practices for pediatric medications, and improved surveillance systems. Health authorities are warning of criminal negligence in pharmaceutical production while emphasizing that most childhood coughs don't require medication.
Dangerous Diethylene Glycol Contamination Found in 'Coldrif' Cough Syrup: Health Ministry Confirms Safety Risk
Oct 04, 2025 05:44 pm CST
The Health Ministry has confirmed that 'Coldrif' cough syrup manufactured by Sresan Pharma in Tamil Nadu contains diethylene glycol (DEG) exceeding safe limits. This industrial solvent can cause severe kidney damage, liver failure, and death, particularly in children. Both Tamil Nadu and Madhya Pradesh have banned the product while authorities conduct comprehensive investigations at 19 manufacturing sites across six states.