Deadly Cough Syrup Tragedy: How Tamil Nadu Regulatory Failures Led to 23 Children's Deaths
- Date & Time:
- |
- Views: 16
- |
- From: India News Bull
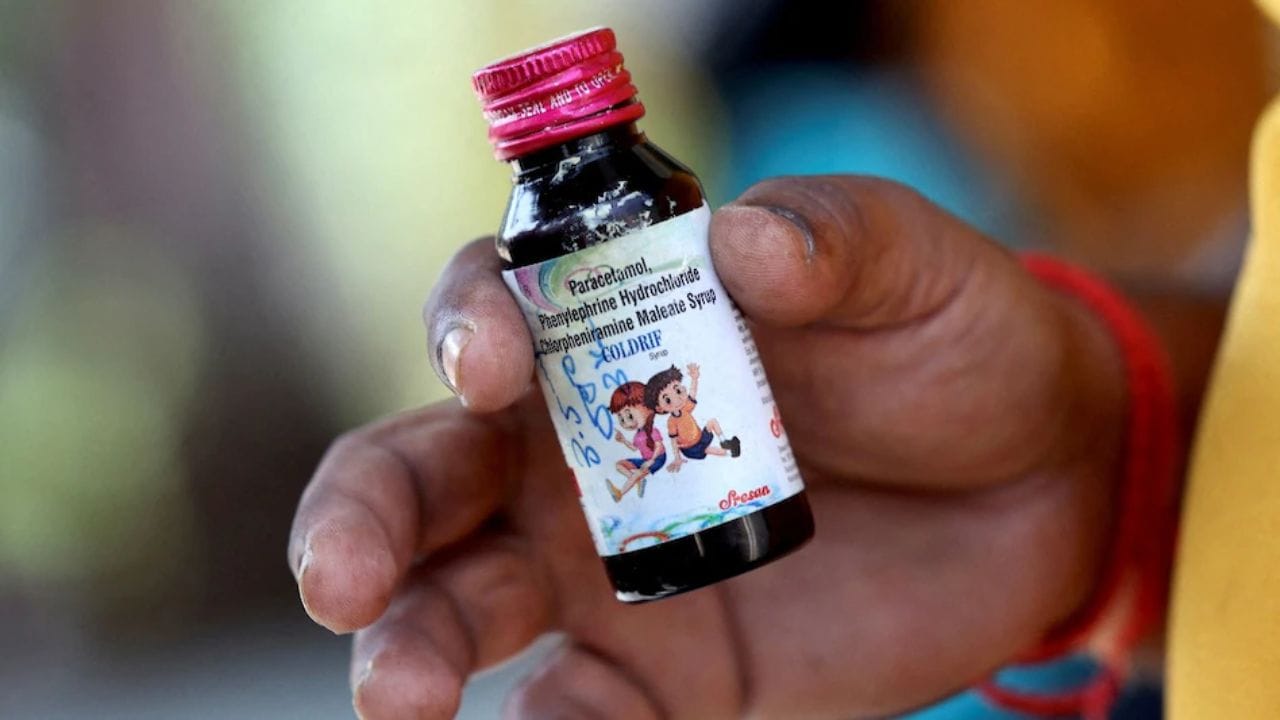
The central drug regulator has identified negligence by Tamil Nadu authorities as the root cause of the tragic cough syrup incident. According to sources, an investigation conducted by the Central Drugs Standard Control Organisation (CDSCO) determined that shortcomings by the state drug controller contributed to the deaths of at least 23 children in Madhya Pradesh who consumed contaminated cough syrup manufactured by a Tamil Nadu-based company.
Sources report that the state drug regulator disregarded regulations and failed to implement the centre's recommendations. They neither took prompt action against the syrup manufacturer nor maintained adequate supervision, which allowed the toxic syrup to enter the market and subsequently caused the children's fatalities.
The CDSCO investigation uncovered that Sresan Pharmaceuticals, producer of the harmful Coldrif syrup, had never undergone an audit by any regulatory agency. Furthermore, the company had not registered on the mandatory central portal for drug manufacturers. Following several days of evading authorities, Ranganathan Govindan, the company's owner, was apprehended two days ago.
Central regulators have criticized Tamil Nadu authorities for permitting the company to function for decades without obtaining WHO-Good Manufacturing Practice (GMP) certification. Sources indicate the central regulator also alleged that the state government was uncooperative with the central investigation team, and the drug controller withheld necessary information.
According to sources, state authorities failed to properly monitor the pharmaceutical company's activities despite having granted it production authorization.
Sresan received its initial license in 2011, which was subsequently renewed in 2016. Earlier this month, state authorities declared the Coldrif cough syrup adulterated after samples revealed the presence of diethylene glycol (DEG), a toxic substance known to cause severe damage to the kidney, liver, and nervous system in humans.
The company's manufacturing facility was discovered to be in deplorable condition. A recent inspection at the Kancheepuram factory revealed unbilled containers of DEG, and evidence that the company was incorporating 46-48% DEG in Coldrif, drastically exceeding the permissible limit of only 0.1%.
Sources citing the CDSCO findings stated that the Tamil Nadu drug controller never communicated this information to the central government.
The centre had implemented a national data portal to enhance pharmaceutical monitoring, but despite registration being mandatory, Sresan never complied. In October 2023, a Google form was distributed to all states requesting pharmaceutical information. This directive was emphasized during monthly review meetings and state FDA (food and drug administration) gatherings. However, Sresan disregarded these requirements and also failed to register on the SUGAM portal, which is legally mandatory.
While state regulators are responsible for ensuring pharma companies register on central portals, the CDSCO determined that the Tamil Nadu Drug Control Department failed in this duty, according to sources.
Sresan Pharma was also excluded from all CDSCO audits, despite state regulators being responsible for auditing all pharmaceutical companies within their jurisdiction.
Sources revealed that earlier this month, the Tamil Nadu government conducted an audit of Sresan Pharma at the request of Madhya Pradesh authorities but did not inform the centre. On October 3, when a central team arrived in Kancheepuram to inspect the factory, the state drug officer failed to appear despite repeated contact attempts.
That same night, state authorities announced the discovery of 48% DEG in the syrup and sealed the factory.
Source: https://www.ndtv.com/india-news/central-drug-regulator-blames-tamil-nadu-lapses-for-syrup-tragedy-sources-9435628