Sresan Pharmaceuticals
Deadly Commission: Doctor Received Rs 2.5 For Each Bottle of Toxic Cough Syrup That Killed 23 Children
Oct 14, 2025 07:33 pm CST
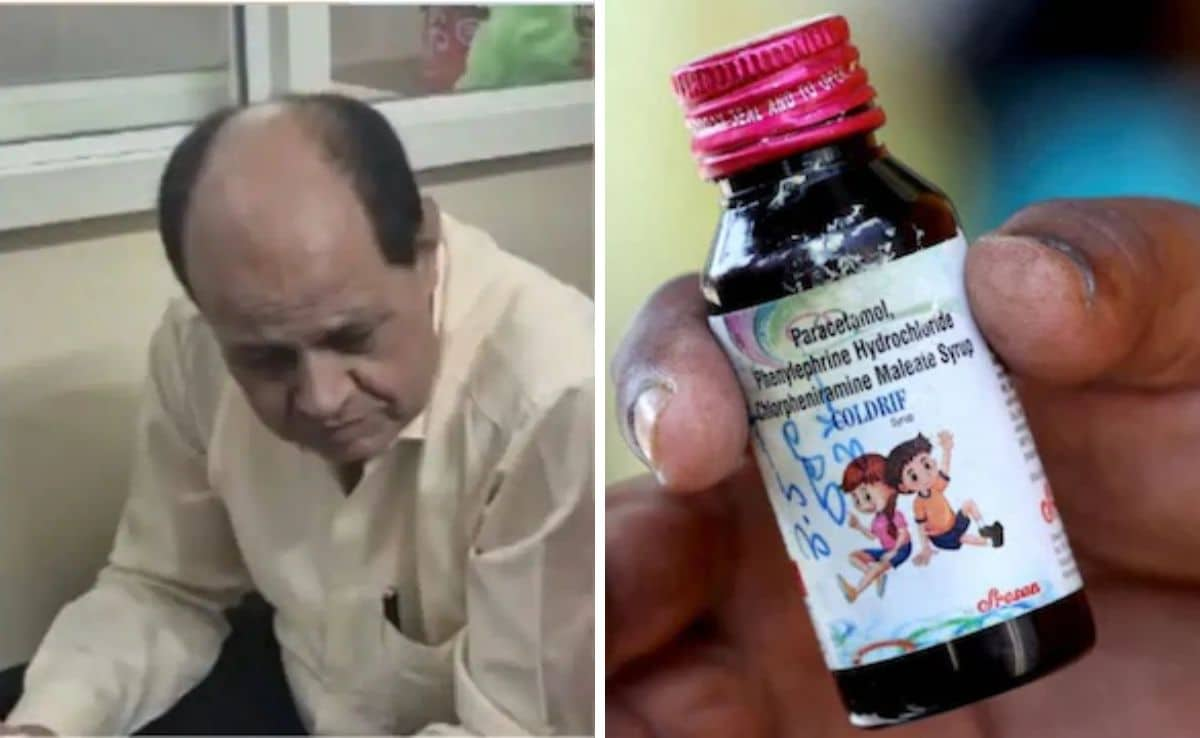
An investigation into the Madhya Pradesh cough syrup tragedy reveals a disturbing pharmaceutical kickback scheme where Dr. Praveen Soni received a 10% commission for prescribing Coldrif syrup that contained toxic diethylene glycol, resulting in 23 children's deaths. The case exposes broader concerns about pharmaceutical corruption and medical ethics in India's healthcare system.
Tamil Nadu Authorities Revoke License of Pharmaceutical Firm Linked to Fatal Cough Syrup Cases
Oct 14, 2025 12:31 am CST
The Tamil Nadu Drug Control department has revoked Sresan Pharmaceuticals' manufacturing license following the discovery of over 350 serious violations at their facility. Their 'Coldrif' cough syrup, contaminated with diethylene glycol, caused multiple child deaths in Madhya Pradesh and Rajasthan. The Enforcement Directorate has launched raids at seven Chennai locations as investigations expand into potential financial irregularities related to the distribution of the toxic medication.
ED Raids Premises of Pharmaceutical Firm Linked to Fatal Cough Syrup: 20 Children Dead from Contaminated Medication
Oct 13, 2025 12:14 pm CST
The Enforcement Directorate has raided multiple premises of Sresan Pharmaceuticals, manufacturer of 'Coldrif' cough syrup that contained toxic diethylene glycol and caused at least 20 children's deaths. The investigation revealed over 350 violations at the company's facility, prompting state bans and WHO concerns about India's regulatory gaps in drug safety screening.
Deadly Cough Syrup Tragedy: How Tamil Nadu Regulatory Failures Led to 23 Children's Deaths
Oct 11, 2025 02:32 pm CST
An investigation by India's Central Drugs Standard Control Organisation reveals critical regulatory failures by Tamil Nadu authorities that allowed a pharmaceutical company to produce toxic cough syrup containing 48% diethylene glycol, resulting in the deaths of 23 children in Madhya Pradesh. The manufacturer operated for decades without proper certification, audits, or registration on mandatory regulatory portals.
Deadly Cough Syrup Scandal: 23 Children Die as CAG Report Reveals Systemic Failures in Drug Testing
Oct 10, 2025 04:06 pm CST
A CAG audit has revealed critical failures in drug testing procedures following the deaths of 23 children in Madhya Pradesh from toxic cough syrup. The report highlights significant shortfalls in Tamil Nadu's pharmaceutical inspection system, where manufacturer Sresan Pharmaceuticals was found adding dangerous levels of diethylene glycol to their Coldrif syrup, far exceeding safety limits. This regulatory negligence has sparked a heated blame exchange between state governments.
Deadly Coldrif Cough Syrup Tragedy: 23 Children Dead as Madhya Pradesh CM Blames Tamil Nadu for Regulatory Failures
Oct 10, 2025 02:13 am CST
The Coldrif cough syrup tragedy has claimed 23 children's lives in Madhya Pradesh, with Chief Minister Dr. Mohan Yadav directly blaming Tamil Nadu's government for regulatory failures. Police have arrested the 75-year-old owner of Sresan Pharmaceuticals, while authorities investigate how the toxic medication reached vulnerable patients across multiple districts.
Coldrif Cough Syrup Manufacturer Arrested: Owner Charged in Fatal Adulteration Case Linked to Children's Deaths
Oct 09, 2025 01:36 pm CST
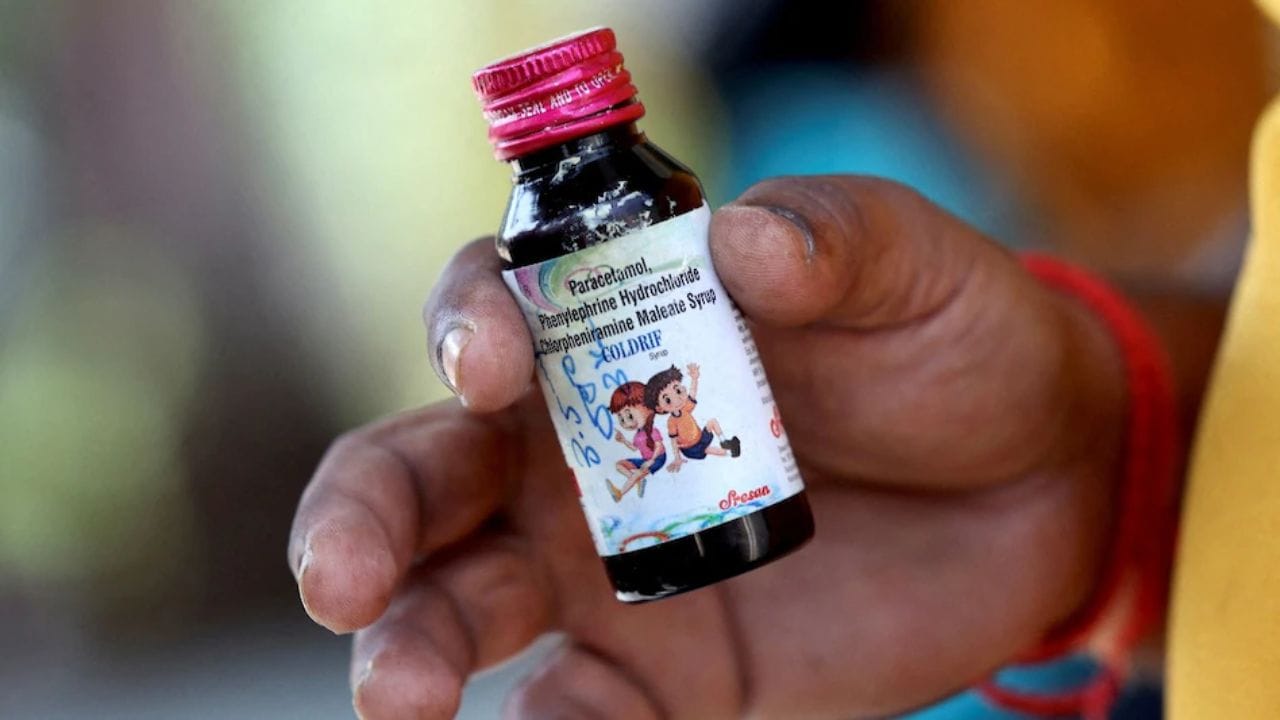
The owner of Sresan Pharmaceuticals, Ranganathan Govindan, has been arrested for manufacturing adulterated Coldrif cough syrup linked to at least 20 children's deaths across multiple Indian states. Investigations revealed the syrup contained dangerous levels of diethylene glycol (DEG), a toxic substance used in printing ink and glue manufacturing, at 46-48% against the permitted 0.1%. Nine states have banned the product as authorities expand their investigation into the deadly pharmaceutical scandal.
FAIMA Urges Health Ministry to Form Central Committee to Investigate Fatal Contaminated Cough Syrup Cases
Oct 08, 2025 09:07 pm CST
The Federation of All India Medical Association (FAIMA) has called for a central investigation committee to examine the deaths of over 20 children in Madhya Pradesh and Rajasthan linked to contaminated Coldrif cough syrup. The medical body emphasized the need for nationwide testing of pediatric medications, improved regulatory coordination, and protection of physicians from premature blame while ensuring pharmaceutical safety standards are enforced.
Deadly Oversight: Coldrif Cough Syrup Contains Banned Ingredients Linked to Multiple Child Deaths in India
Oct 08, 2025 02:13 pm CST
An investigation reveals that Coldrif cough syrup, linked to 19 children's deaths in Madhya Pradesh, contains ingredients banned for children under four years and dangerous levels of diethylene glycol. Despite a 2023 government directive requiring warning labels and WHO-GMP certification requirements following global child fatalities from contaminated medications, the manufacturer Sresan Pharmaceuticals continued production with 480 times the permitted toxic chemical level without proper certification.
Deadly Contamination: How Substandard Manufacturing Led to Fatal Cough Syrup Poisoning of 16 Children
Oct 06, 2025 10:50 pm CST
An investigation into the fatal Coldrif cough syrup reveals shocking manufacturing conditions at Sresan Pharmaceuticals, where industrial chemicals replaced pharmaceutical-grade ingredients, leading to 16 children's deaths in Madhya Pradesh. Inspectors found 39 critical and 325 major violations, including untested raw materials, untrained staff, and toxic levels of diethylene glycol—nearly 500 times above permissible limits.