drug safety
US FDA Confirms Toxic Cough Syrups Linked to Child Deaths in India Not Imported to United States
Oct 11, 2025 09:44 am CST
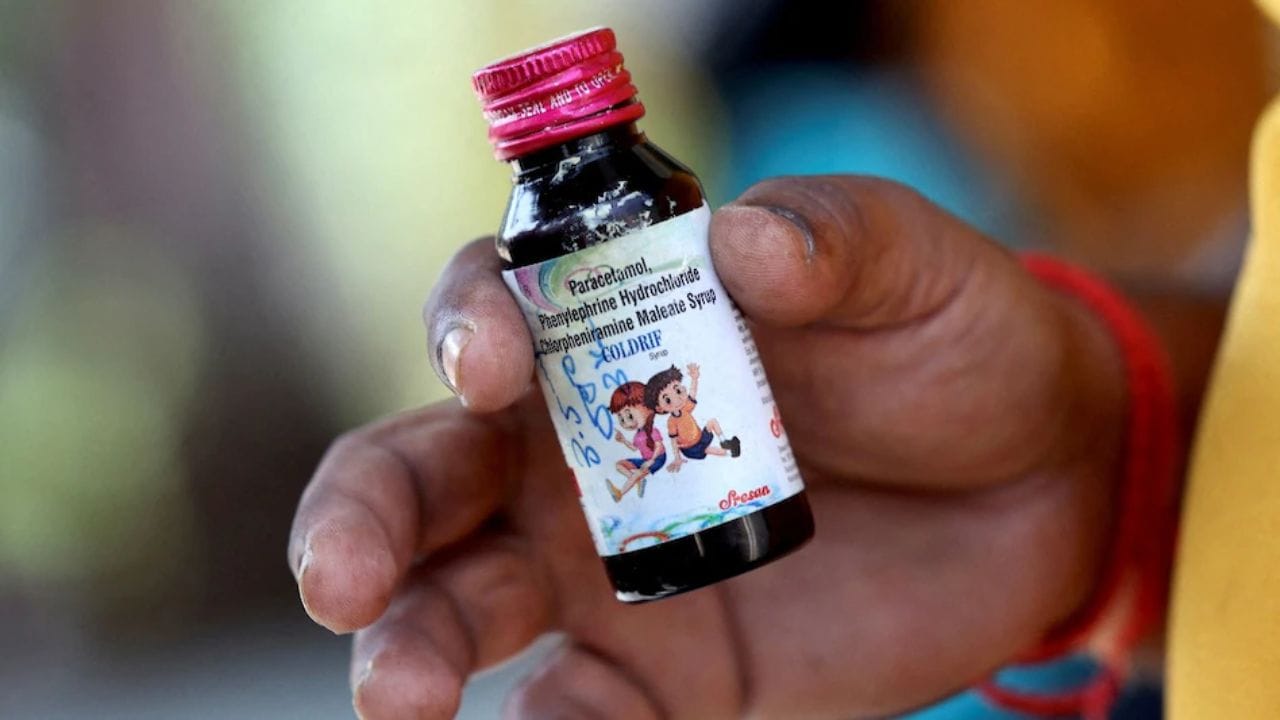
The US Food and Drug Administration has confirmed that toxic cough syrups responsible for 17 children's deaths in India were not exported to the United States. Indian authorities banned the Coldrif brand after tests revealed diethylene glycol levels 500 times above the permissible limit, while the WHO highlighted India's "regulatory gap" in screening medications sold locally.
Deadly Negligence: How 20 Children Died from Toxic Cough Syrup While Madhya Pradesh's Drug Testing System Failed
Oct 08, 2025 11:51 pm CST
In Madhya Pradesh, India, a catastrophic breakdown in the drug regulatory system resulted in 20 children's deaths from toxic cough syrup Coldrif. This tragedy exposes critical failures including drug samples sent by ordinary post, delayed testing, premature safety declarations, and an overwhelmed infrastructure with unused mobile testing units. While Tamil Nadu acted swiftly to ban the dangerous medication, Madhya Pradesh's bureaucratic delays allowed the toxic syrup to claim more lives, revealing systemic issues that remain unresolved even as seven children continue fighting for survival.
Deadly Oversight: Coldrif Cough Syrup Contains Banned Ingredients Linked to Multiple Child Deaths in India
Oct 08, 2025 02:13 pm CST
An investigation reveals that Coldrif cough syrup, linked to 19 children's deaths in Madhya Pradesh, contains ingredients banned for children under four years and dangerous levels of diethylene glycol. Despite a 2023 government directive requiring warning labels and WHO-GMP certification requirements following global child fatalities from contaminated medications, the manufacturer Sresan Pharmaceuticals continued production with 480 times the permitted toxic chemical level without proper certification.
India's Health Ministry Responds to Child Deaths from Contaminated Cough Syrup: Urgent Regulatory Actions and Safety Measures
Oct 05, 2025 11:15 pm CST
Following the deaths of 14 children in Madhya Pradesh and Rajasthan linked to contaminated cough syrup, India's Health Ministry has implemented emergency measures to enhance pharmaceutical oversight. The tainted Coldrif cough syrup contained diethylene glycol, prompting stricter enforcement of manufacturing standards, rational prescription practices for pediatric medications, and improved surveillance systems. Health authorities are warning of criminal negligence in pharmaceutical production while emphasizing that most childhood coughs don't require medication.
Deadly Negligence: 11 Children Die from Toxic Cough Syrup in Madhya Pradesh While Officials Delay Action
Oct 05, 2025 05:07 pm CST
An NDTV investigation reveals systemic failures in Madhya Pradesh's health system after 11 children died from Coldrif cough syrup contaminated with 48.6% Diethylene Glycol. While Tamil Nadu authorities identified and banned the toxic medication within 24 hours, Madhya Pradesh officials delayed action for weeks, conducted no autopsies, and took holidays during the crisis, raising serious questions about accountability and public health safety.