CDSCO investigation
Deadly Cough Syrup Tragedy: How Tamil Nadu Regulatory Failures Led to 23 Children's Deaths
Oct 11, 2025 02:32 pm CST
An investigation by India's Central Drugs Standard Control Organisation reveals critical regulatory failures by Tamil Nadu authorities that allowed a pharmaceutical company to produce toxic cough syrup containing 48% diethylene glycol, resulting in the deaths of 23 children in Madhya Pradesh. The manufacturer operated for decades without proper certification, audits, or registration on mandatory regulatory portals.
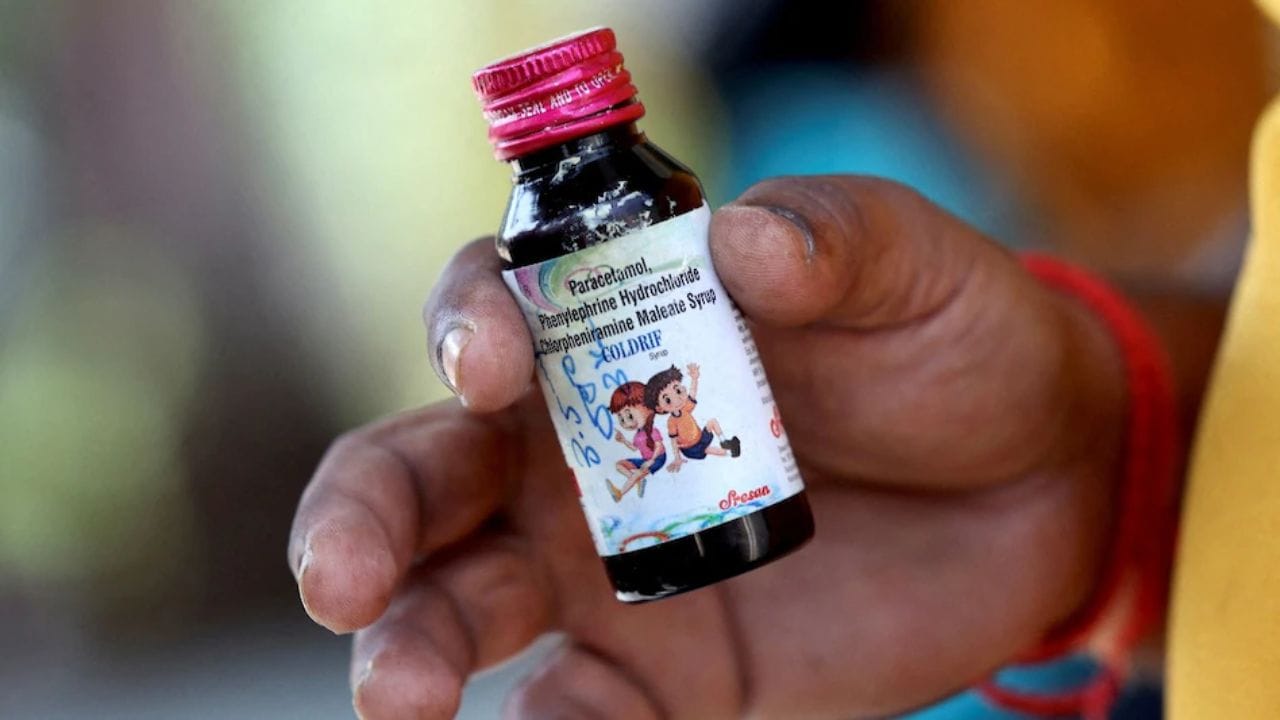
364 Violations Discovered at Sresan Pharma Following Deadly Cough Syrup Incident: DEG Contamination Claims 20 Children's Lives
Oct 09, 2025 09:04 pm CST
An investigation into Sresan Pharma revealed 364 violations at their Tamil Nadu facility following a tragic incident where contaminated cough syrup containing excessive diethylene glycol resulted in at least 20 child fatalities in Madhya Pradesh. The CDSCO is now launching nationwide inspections of cough syrup manufacturers while several states implement stricter regulations for pediatric cough medication dispensing.