drug safety regulations
Investigation Reveals Safety Lapses and Regulatory Failures in Fatal Cough Syrup Contamination Case
Nov 21, 2025 04:14 pm CST
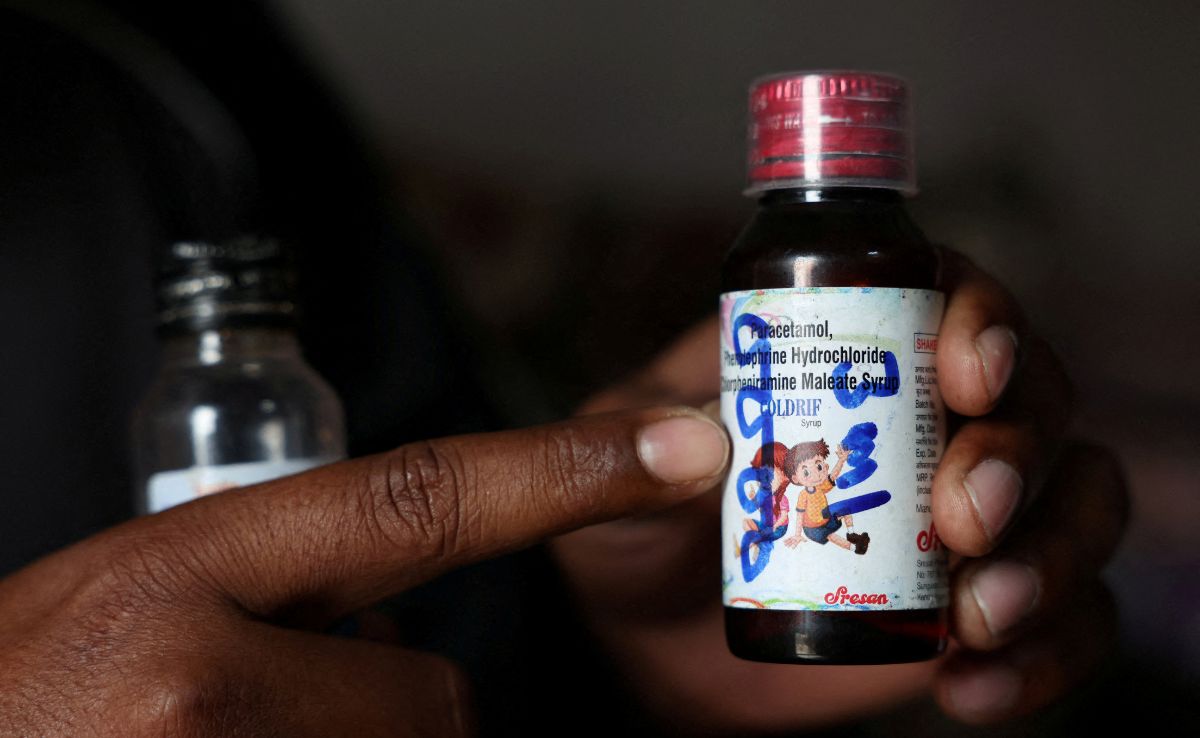
An exclusive investigation into the deaths of 24 children from contaminated cough syrup has uncovered serious safety breaches in India's pharmaceutical supply chain. The report details how improper handling of propylene glycol solvent, unauthorized repackaging by unlicensed distributors, and inadequate regulatory oversight may have allowed toxic diethylene glycol to contaminate Coldrif cough syrup, raising new concerns about quality control in India's $50 billion pharmaceutical industry.
364 Violations Discovered at Sresan Pharma Following Deadly Cough Syrup Incident: DEG Contamination Claims 20 Children's Lives
Oct 09, 2025 09:04 pm CST
An investigation into Sresan Pharma revealed 364 violations at their Tamil Nadu facility following a tragic incident where contaminated cough syrup containing excessive diethylene glycol resulted in at least 20 child fatalities in Madhya Pradesh. The CDSCO is now launching nationwide inspections of cough syrup manufacturers while several states implement stricter regulations for pediatric cough medication dispensing.