pharmaceutical quality control
Investigation Reveals Safety Lapses and Regulatory Failures in Fatal Cough Syrup Contamination Case
Nov 21, 2025 04:14 pm CST
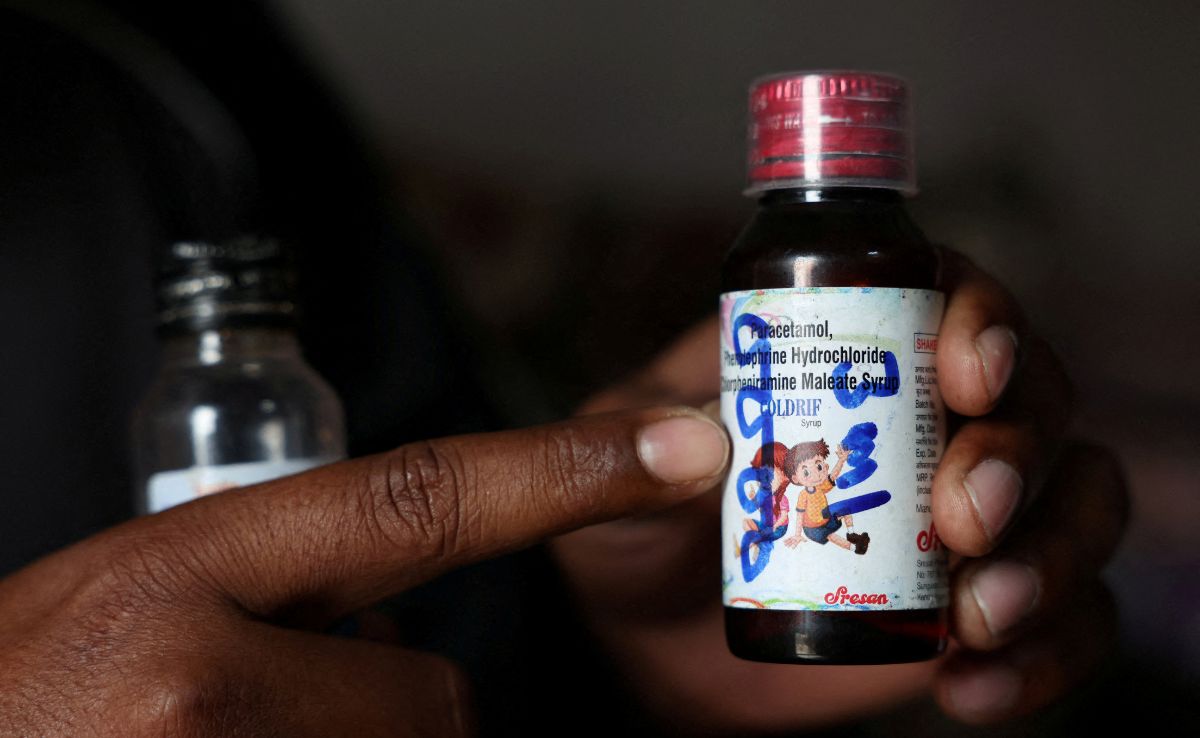
An exclusive investigation into the deaths of 24 children from contaminated cough syrup has uncovered serious safety breaches in India's pharmaceutical supply chain. The report details how improper handling of propylene glycol solvent, unauthorized repackaging by unlicensed distributors, and inadequate regulatory oversight may have allowed toxic diethylene glycol to contaminate Coldrif cough syrup, raising new concerns about quality control in India's $50 billion pharmaceutical industry.
CAG Audit Reveals Critical Failures in Madhya Pradesh's Medical System Before Deadly Syrup Tragedy
Oct 10, 2025 06:50 pm CST
A comprehensive CAG audit report exposed severe mismanagement in Madhya Pradesh's medicine storage systems and healthcare infrastructure one year before contaminated Coldrif cough syrup caused 23 children's deaths. The report highlighted the absence of mandatory annual physical verification at multiple hospitals including CIMS Chhindwara, expired medications worth Rs 1.08 crore, improper storage practices, and critical healthcare worker shortages reaching up to 92% in some facilities, revealing systemic failures in India's pharmaceutical safety protocols.